Balancing Net Ionic Redox Equations
The nitrogen atoms and the oxygen atoms are difficult to balance by inspection so we will go to Step 3. Balance the following equations of redox reactions.
Oxidation Reduction Reactions Redox
Balancing a Net Ionic Redox Equation step-by-step 1.

. Ag HNO3 AgNO3 NO2 H2O Ag2S HNO3 konc AgNO3 NO2 S H2O Ag2S HNO3 dil AgNO3 NO S H2O As HNO3 H2O H3AsO4 NO As NO3- As2O5 NO. View Balancing Net Equations for Redox Reactionspdf from CHEMISTRY 031 at Malayan Colleges Laguna. Use the rules to assign oxidation numbers to all atoms in the equation.
Solution for Balance the net ionic redox equations by the half-reaction method. In the oxidation number method you determine the oxidation numbers of all atoms. 5 A more detailed discussion about balancing this equation can be found here.
Full reaction balanced in acid. Identify which atoms are oxidized and which are reduced. Textbook Solutions Expert Tutors Earn.
Cu s HNO 3 aq Cu NO32aq NO g H2O l Solution. Full reaction balanced in acid. Then you balance by making the electron loss equal the electron gain.
Balance the following redox equation using either the inspection technique or the oxidation number method. Any help would be greatly appreciated. Here it is in all its glory.
Balancing a redox equation in basic solution. You just have to insert the equation and the calculator will display oxidation and reduction. CH 3 CH 2 OH H 2 O CH 3 COOH 4H 4e - Cr 2 O 72- 14H 6e - 2Cr 3 7H 2 O.
Identify all of the phases in your answer. Check that both sides of the equation have the same kind and number of atoms as well as the same charges. Ive been working on this problem for about 30 minutes and Im still unable to achieve an answer.
Add 6 electrons to the left-hand side to give a net 6 on each side. In order to understand how this is done consider the balanced equation for the reaction of ironII nitrate and. Write balanced net ionic equations for the reaction that takes place between H2O2 aq and MnO-4 all numbers are subscripts of the respective element with the.
It winds up with the equation balanced in basic solution. Then you multiply the atoms that have changed by small whole numbers. Part A VO2aqHaqClO2aqVO2aqClO2gH2Ol Express your answer as a net ionic equation.
6 I once saw an unusual method to balancing this particular example equation. Balance the Redox Reaction in Acidic Medium by Half Reaction Method. ELECTROCHEMISTRY Electrochemistry BALANCING NET IONIC EQUATIONS FOR REDOX REACTIONS Writing ionic.
Be sure to check that the atoms and the charge are balanced. The balancing redox reactions calculator tells whether a reaction is actually a redox reaction or not. Finally for the specified chemical equation a window will pop with the output.
Any help would be greatly appreciated. The net ionic equation is thus obtained. Ive been working on this problem for about 30 minutes and Im still unable to achieve an answer.
Balancing Redox Equations 2 Balance each of the following net ionic equations for a redox reaction. The only sure-fire way to balance a redox equation is to recognize the oxidation part and the reduction part. Balancing a Net Ionic Redox Equation step-by-step 1.
Add the two half-reactions together and cancel out common terms Balance the following redox equation by the oxidation number methode in acidic solution. Draw a line connecting the atoms involved in oxidation and another line connecting the atoms involved in reduction. Similarly it also balances the number of charges ions and atoms at both sides of the equation to help you understand the reaction more easily.
Now all that needs balancing is the charges. Introduction to acidbase reactions. Ad Browse Discover Thousands of Science Book Titles for Less.
Check that both sides of the equation have the same kind and number of atoms as well as the same charges. Cr 2 O 72- 14H 6e - 2Cr 3 7H 2 O Combining the half-reactions to make the ionic equation for the reaction What we have so far is. Balancing a redox equation in acidic solution.
Write balanced net ionic equations for the reaction that takes place between H2O2 aq and MnO-4 all numbers are subscripts of the respective element with the. Balancing net ionic redox equations The simplest way to express a redox reaction is an equation that shows only the oxidation and reduction processes. Cu NO32 KI CuI I2 KNO32 Ag2SO4 KCl AgCl K2SO4 Co NO32 K3PO4 Co3 PO42 KNO3 K3PO4 Sr NO32 Sr3 PO42 KNO3 Ba NO32 K2CrO4 BaCrO4 KNO3 NaNO3 KOH NaOH KNO3 Pb NO32 ZnI2 PbI2 Zn NO32 Na2CO3 CaCl2 NaCl CaCO3 Fe NO32 NaOH Fe OH2 NaNO3 SnSO4 NH42S SnS NH42SO4.
__PbO2 __Cl __Pb2 __ClO3. Cr 2 O 7 2 Cl --- Cr 3 Cl 2 O 2 there is a minimum of 2 Crs 2Cr 6 6e --- 2Cr 3. __CrO42 __C6H6 __Cr3 __CO2.
This final check confirms that the equation is perfectly balanced in terms of atoms and charges. Solution for Balance the net ionic redox equations by the half-reaction method.
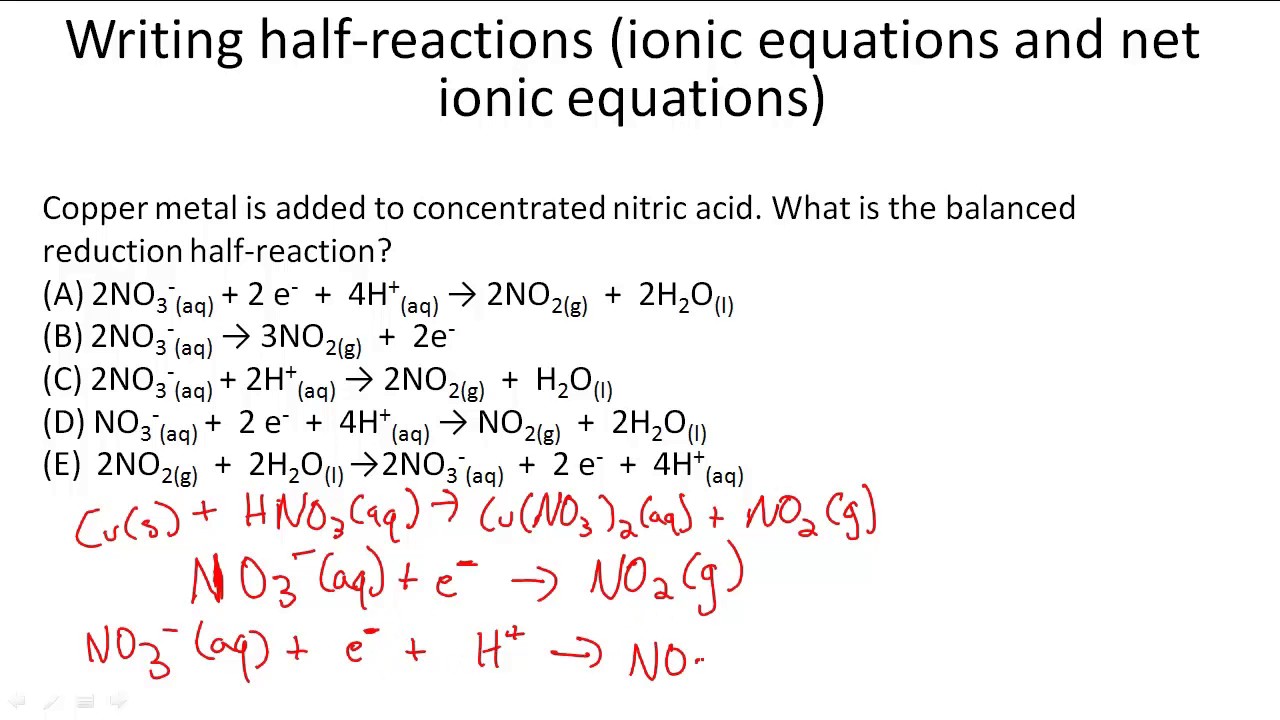
Writing Half Reactions Ionic Equations And Net Ionic Equations Youtube
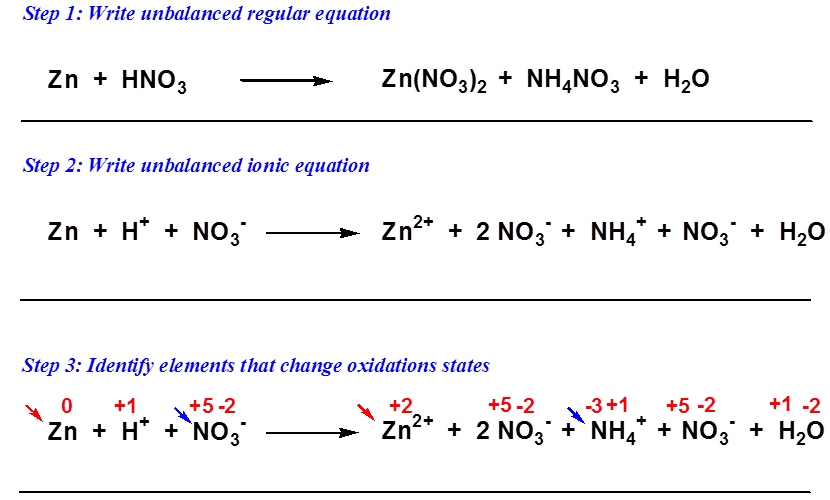

No comments for "Balancing Net Ionic Redox Equations"
Post a Comment